|
|
Capillaster Multiradiatus
(Linnaeus 1758)
|
|
Sarah Anne McCulloch 2018
|
|
|
|
Summary | |
Capillaster multiradiatus is a member of Echinodermata – it’s related to sea stars, sea urchins, and sea cucumbers. It belongs to Comatulida, the stalkless feather stars in class Crinoidea. While many feather stars look incredibly similar, a small central body surrounded by a radial of feather-like arms, C. multiradiatus has a unique skeletal pattern to set it apart from other species (Ruppert, E. E. et. al, 2004). More explained on that point in the Phylogeny section.
This species can be found in number oceans and seas in the tropical Indo-western Pacific. A cryptic, nocturnal species, this coral reef dwelling animal hides under coral boulders or in small crevices during the day, and only emerges onto the outer reef edges at night to feed.
Key features of this family include:
- Loss of the stalk and adaption of cirri
- Use of appendages for suspension feeding
- A water vascular system that supports feeding, locomotion, respiration, and circulation
- Special structures, known as mutable collagenous tissues, that permit a mainly sedentary lifestyle
- Various strategies to withstand predation
Many species of crinoids play hosts to a wide variety of commensals. The host-specificity of these ectosymbionts is still unknown, so we have conducted an experiment to test whether Capillaster multiradiatus is a preferred host to certain inhabitants.
|
|
|
Physical Description | |
Most species within the Capillaster family have very similar general characteristics, yet within C. multiradiatus, individuals can differ greatly. Arm and cirri number and length, colour, or size can all fluctuate. Other specimens of this species have been found entirely white or pale yellow (Messing, C. G. and Tay, T. S. 2016), and others dark red or light brown (Summers, M. M. et. al 2017).
Size
Individuals of this species are medium-sized crinoids, with arms an average of 65-70mm at full length, but greatest length can be up to 95mm (Marshall, J. I. and Rowe, F. W. E. 1981). The tribe, Capillasterini, has a crown that consists of a maximum number of 110 arms (Summers, M. M. et. al 2017), but usually C. multiradiatus has only 12-35 arms (Clark, A. H. 1921, Messing, C. G. and Tay, T. S. 2016). Along each arm are small, feather-like appendages called pinnules.
Figure 1: the aboral side of C. multiradiatus showing the cirri, arms, and pinnules.
The centrodorsal is a thick, disc-shaped modified ossicle from which the arms radiate, and is on average 4mm in diameter. Since this family discards its postlarval stalk, to hold stable on the rocks and coral it uses a ring of cirri that arise from the aboral side of the centrodorsal. These cirri are usually about 15 to 18mm long, and number from 15 to 28 (Clark, A. H. 1921). This specimen had 12 arms (plus 2 small arms currently growing) with an average length of 54mm (not including the 2 growing arms), with many of its arms having lost the ends due to handling in the lab. It had 19 cirri with 16 segments, with the average cirri length at 12mm.
Colour
As stated previously, C. multiradiatus can vary greatly in colour, ranging from nearly black, differing shades of brown, deep violet with some having patterning and colouration on the arms and pinnules. This particular specimen was a deep reddish-brown colour, with a bright yellow aboral surface.
Figure 2: the oral side of C. multiradiatus demonstrating the reddish-brown colour of the specimen
|
|
|
Ecology |
Local Distribution and Habitats | |
Distribution
The individual Capillaster multiradiatus specimen was collected from the reef ecosystem situated along the rock wall at the Gold Coast Seaway, Queensland, Australia, as seen in figure 3. While this location is quite southerly to find this particular species, other specimens have been found off Gladstone, Queensland (gbif.org, 2018). Individuals of this species are found as far south as 29.8 South in Western Australia (Online Zoological Collections of Australian Museums. 2005), so it is not uncommon for them to be present in subtropical waters.
|
|
|
Commensalism and Predation | |
Commensalism
Crinoids shelter a wide variety of species of marine organisms in many symbiotic relationships (Deheyn, D. D et. al, 2006). Some of the phylum and families that live on or within crinoids include Myzostomida, Decapoda, Galatheidea, Gastropoda, Polychaeta (Deheyn, D. D et. al, 2006).
The most common two reasons for animals to live symbiotically with crinoids are the ability to hide from predation among the complex morphological structures of feather stars, and the similarity of prey type and feeding mechanism, meaning food capture is easy for the symbiont (Deheyn, D. D et. al, 2006). Many symbiotic inhabitants feed on the plankton that has been trapped by their crinoid hosts (Fabricius, K. E. and Dale, M. B. 1993). Colour similarity between the host crinoid and symbiont have been recorded (Deheyn, D. D et. al, 2006), with many symbionts that live externally on the crinoids being well camouflaged (Fabricius, K. E. and Dale, M. B. 1993).
Crinoid Host-Selection by a Crustacean
Commensals living on or in crinoids have been shown to have high host-specificity (Fishelson, L., 1974). A species of squat lobater known as the feather star squat lobster, Allogalathea elegans, has shown in many studies to select Capillaster multiradiatus as its primary host (Baeza, J. A. 2011, Encyclopedia of Life, 2018).
When this specimen of C. multiradiatus was collected from the Gold Coast, an individual A. eleganswas found hiding amongst the feather stars’ arms. Myself, along with colleague Michael, wanted to investigate whether this squat lobster would continuously select C. multiradiatus as its host when given a variety of host options.
Two separate experiments were run to investigate the decision time for host selection, and the frequency of selection. The hypotheses for these experiments were were:
- Decision time: Allogalathea elegans would take the least amount of time to select Capillaster multiradiatus as a host, when only given one choice, and take the longest time to chose the rock as the host
- Selection frequency: Allogalathea elegans select Capillaster multiradiatus as a host more frequently than any other species/object, when able to chose between many hosts, and select the rock least frequently
The decision time experiment was run so that A. elegans would be placed into a container of water with only one host species/object, as shown in figure 5. There were four separate treatments, one with C. multiradiatus, one with only rock, and two sepearte treatments, both with a different Crinoid species, taken from the same location as C. multiradiatus; Lamprometra palmate, and Comaster schlegelii. This is illustrated in the figure below. Time was recorded from when the lobster was released into the water, to when it was in contact with the host for 5 seconds. Time was then average between the 5 trials of each treatment, to give an average time score for each host.
|
|
|
|
Life History and Behaviour |
Life History | |
Reproduction
All crinoids are gonochoric, whereby the male and female reproductive organs are in separate individuals (Vail, L. 1987). The sexes are indistinguishable apart from gonads with mature gametes which can sometimes be recognised as ovaries or testes (Byrne, M., and O'Hara, T. 2017). The gonads are situated on the ambulacral side of the genital pinnules (Byrne, M., and O'Hara, T. 2017), and all develop asynchronously, with those in the lower quarter of the arm maturing first (Rutman, J. L., and Fishelson, L. 1985). Gonads consist of a genital tuble enclosed in a hemal sinus, which is surrounded by the genital coelom (Ruppert, E. E. et al. 2004).
The reproductive cycle of each gonad includes six stages. Oogonia and spermatoogonia differentiate from the innermost layer of the gonad wall, and as spermatozoa matures and ripens it accumulates in the lumen. Ripening of male gametes is synchronous, while in females ripening is asynchronous (Rutman, J. L., and Fishelson, L. 1985). Along one on an individual, the gonads are in differing stages of development, with the most proximal gonads usually being the most advanced (Rutman, J. L., and Fishelson, L. 1985). Gametes are fertilised externally in most crinoids, and most broadcast their gametes (Byrne, M., and O'Hara, T. 2017). Capillaster multiradiatus has been observed to spawn just after dark (Vail, L. 1987). Spawning occurs by rupturing of the pinnules walls, and gametes then released into the water (Ruppert, E. E., Fox, R. S. and Barnes, R. D. 2004).
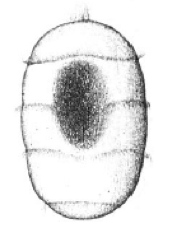
Development
All crinoids have short-lived, non-feeding larvae, beginning with a non-feeding doliolaria that is barrel-shaped and has four or five hoop-like ciliated bands and an anterior apical tuft (Ruppert, E. E. et al. 2004), as shown in figure 6. For a short time, it is free-swimming, but undergoes many morphological changes until settlement on the bottom (Rutman, J. L., and Fishelson, L. 1985). They attach to the substrate via a glandular midventral depression, located near the apical tuft. Metamorphosis then occurs an a benthic, non-feeding, stalked crinoid develops (McEdward, L. R., and Miner, B. G., 2001). Comatulids continue metamorphosis into a stalked, feeding sessile stage, known as a pentacrinoid. Up to several months later, during which time the cirri have formed, the crown releases from the stalk and the young adult comatulid begins living a free-existence (Ruppert, E. E. et al. 2004).
Figure 6: Doliolaria larvae of a crinoid species. Image source: (McEdward, L. R., and Miner, B. G., 2001)
|
|
|
Behaviour | |
Feeding
Crinoids have been labelled as passive, non-selective suspension feeders that rely on water currents to bring food to them (Byrne, M., and O'Hara, T. 2017, Meyer, D. L. 1982). However, they do play an active role in modifying their arm and pinnule posture, aligning their arm fans to be normal to the current, and mobile feather stars can seek areas with better conditions for feeding (Byrne, M., and O'Hara, T. 2017). Comatulids anchor their cup to the substrate and extend their arms in a half circular ‘fan’ (Baumiller, T. K., 2008). Capillaster multiradiatusdoesn’t form a true filtration fan, but still extends its arms from the central point and responds to tidal current flows over the reef (Meyer, D. I., 1979).
During feeding the arms are outstretched with the tube feet upright (Ruppert, E. E. et al. 2004). The tube feet bear mucus-secreting papillae, which assist in food capture (Rutman, J., and Fishelson, L., 1969), and occur in a series of triplets (Ruppert, E. E. et al. 2004). The primary tube foot is the longest, and all primary feet on one pinnule create a food-catching mesh. From the feet, food is transferred to the ambulacral groove and to the mouth to be ingested (Ruppert, E. E. et al. 2004). Its believed that food capture is only limited by the ambulacral groove width and activity of the prey (Meyer, D. L. 1982). However, food particles much larger than groove width in C. multiradiatushave been recorded in stomach contents (Byrne, M., and O'Hara, T. 2017).
The length and spacing of the tube feet has been found to influence position of a species within the reef (Meyer, D. I., 1979). Species with longer and more widely spaced tube feet are more likely to live within in structure of the reef, and hold their arms in a multidirectional posture (Meyer, D. I., 1979). Those with tube feet that shorter and spaced closer together tend to position themselves in exposed positions and form complete filtration fans (Meyer, D. I., 1979).
C. multiradiatusfeeds only at night, so emerges roughly one hour before sunset from its daytime hiding place amongst crevices in the reef (Meyer, D. L. 1982). It feeds at lower levels, along side formations of the reef, and will select its habitat for optimal current conditions (Baumiller, T. K., 2008). The main diet of crinoids includes detritus, benthonic microorganisms, and plankton that is ‘raining down’ (Rutman, J., and Fishelson, L., 1969). One study of the stomach of C. multiradiatusfound the following contents: sediment grains: 32.6%, phytoplankton: 32.5%, Protozoa: 20.1%, Mollusca:2.0%, Crustacea: 1.5%, miscellaneous 3.9% (Meyer, D. L. 1982).
Locomotion
Stalkless comatulids have the ability to move freely, and are capable or swimming or crawling within their habitat (Ruppert, E. E., Fox, R. S. and Barnes, R. D. 2004). Movement is always with the oral surface facing up. To crawl around the substratum, comatulids can lift their body up and move around on the downward-flexed arms. They often use the small, terminal hooks on their arms and pinnules to grasp surfaces (Ruppert, E. E., Fox, R. S. and Barnes, R. D. 2004).
Despite their ability to do so, most movement, particularly swimming, is usually only an escape response (Ruppert, E. E., Fox, R. S. and Barnes, R. D. 2004). The highest observed usual movement is of nocturnal species, such as C. multiradiatus, that move between hiding crevices and feeding areas between day and night.
Video 1: the movement of Capillaster multiradiatus, using only its arms and pinnules
Diel Activity
Capillaster multiradiatus is a nocturnal animal, following a diel activity pattern (Meyer, D. I., 1979). This species hides in amongst reef infrastructure, in crevices during the day (Meyer, D. 1985), then at night will move out into areas suitable for suspension feeding (Baumiller, T. K., 2008). Their activity is regulated by sunset (Fishelson, L., 1974), particularly since they are quite sensitive to light. Yet, arguments have been made that daytime disturbances or predation by fish and other predators could also increase the need for a nocturnal lifestyle (Meyer, D. 1985).
|
|
|
|
Anatomy and Physiology |
External Anatomy | |
Body Form
Crinoids have skeletons that are composed of numerous thick, vertebra-like ossicles, giving their appendages a jointed appearance (Ruppert, E. E. et al. 2004). This skeleton is bound with ligament and muscle, and is organised into three body parts: a cup, that holds most of the vital organs, arms, that area arranged radially around this cup, and a stalk, which elevates the cup above the ground (Baumiller, T. K., 2008).
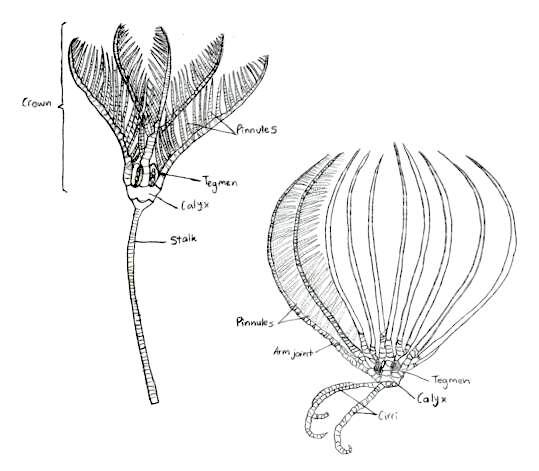 Figure 7: The traditional body form of both the stalked and unstalked crinoids, Capillaster multiradiatus belong to the latter. Picture adapted from Ruppert, E. E. et al. 2004
Figure 7: The traditional body form of both the stalked and unstalked crinoids, Capillaster multiradiatus belong to the latter. Picture adapted from Ruppert, E. E. et al. 2004
The traditional body form of a crinoid includes the stalk that supports the rest of the animal and holds it to the substrate (Ruppert, E. E. et al. 2004), as seen in figure 7. Comatulids however, like Capillaster multiradiatus, only have a stalk during their postlarval stage, and eventually discard it to become free-living (Summers, M. M. et. al 2017). Despite this loss, the most central cirri still remain and form circles at the base of the crown (Fishelson, L., 1974). The crown is pentamerous, meaning its parts are arranged in groups of five, and it is equivalent to the body of other echinoderms (Ruppert, E. E. et al. 2004). The calyx, a heavily calcified cup housing the viscera, and the tegmen, the membranous oral wall, together make up the crinoid disc.
As juveniles, C. multiradiatus starts with a central mouth, but during postlarval development the mouth migrates to a marginal position on the tegmen (Summers, M. M. et. al, 2017, Clark, A. H. 1921). The anus is also present on the tegmen, on the oral (upward facing) side of crinoids, often at the summit of an anal cone, elevating it above the feeding current (Ruppert, E. E. et al. 2004).
Symmetry
Like all species in the phylum Echinodermata, crinoids exhibit pentamerous symmetry, meaning their body can be divided into five similar sections from the central axis (Ruppert, E. E. et al. 2004). However, their larvae display a bilateral symmetry (McEdward, L. R., and Miner, B. G., 2001) which is lost through shifting of the body axis of the individual and through torsion (Ruppert, E. E. et al. 2004).
Arms, Pinnules and Ambulacral Grooves
The arms of Capillaster multiradiatus radiate from the pentamerous crown; whilst primitive species usually only have 5 arms, the base of most crinoids bifurcate and create a total of 10 arms. Further branching along these arm radials can create the appearance of multiple arms (Ruppert, E. E. et al. 2004). C. multiradiatuscan have over 100 arms on one individual. Each arm consists of a series of small articulated plates known as brachials, and a group of these brachials between branchings of the arm are called brachitaxis (Oji, T. and Okamoto. T., 1994).
On each arm is a bilaterial series of pinnately arranged pinnules (Ruppert, E. E. et al. 2004), giving the feather stars’ arms their feather look, and its also its common name. Crinoids also possess modified pinnules that perform different tasks within the individual. Some pinnules are specialised ‘genitals’ that bear the gonads, whilst other pinnules located near the base of the rays are the oral pinnules that can be modified for protecting and cleaning the tegmen (Byrne, M., and O'Hara, T. 2017). The oral pinnules are mechano-receptive and ambulacra or tube feet, and can almost completely cover the disc to protect it (Alender, C.B. et al. 1966). The pinnules also give the species its ‘sticky’ feeling, due to the many proximally-directed hooks on the middle and distal pinnules (Byrne, M., and O'Hara, T. 2017); this is illustrated in more depth in figure 8 below.
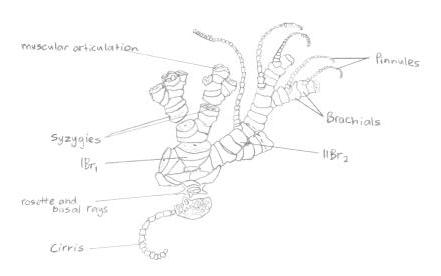
Figure 8: Illustration of a 'semi-exploded' view of the proximal portion of a feather star. Picture adapted from Ruppert, E. E. et. al 2004.
On each arm is a bilaterial series of pinnately arranged pinnules (Ruppert, E. E. et al. 2004), giving the feather stars’ arms their feather look, and its also its common name. Crinoids also possess modified pinnules that perform different tasks within the individual. Some pinnules are specialised ‘genitals’ that bear the gonads, whilst other pinnules located near the base of the rays are the oral pinnules that can be modified for protecting and cleaning the tegmen (Byrne, M., and O'Hara, T. 2017). The oral pinnules are mechano-receptive and ambulacra or tube feet, and can almost completely cover the disc to protect it (Alender, C.B. et al. 1966). The pinnules also give the species its ‘sticky’ feeling, due to the many proximally-directed hooks on the middle and distal pinnules (Byrne, M., and O'Hara, T. 2017).
Five ambulacral grooves, seen in figure 9, are present on the tegmen and these extend onto the oral surface of both the arms and pinnules. These grooves have margins with movable flaps (called lappets), which allow it to be open or closed (Ruppert, E. E. et al. 2004). Food particles are transported down the grooves to the mouth by three tube feet located on the inner side of each lappet (Fatherree, J. W. 2013, Ruppert, E. E. et al. 2004). The arms, pinnules, and ambulacral grooves are all not only used in feeding, but also are used for locomotion (Byrne, M., and O'Hara, T. 2017).

Figure 9: Arrows pointing to the ambulacral gores on the arms of Capillaster multiradiatus
Tube Feet
The tube feet, also known as podia, are small, sticky extensions of the water vascular system located in the ambulacral grooves of echinoderms (Baumiller, T. K., 2008), seen in figure 10. However, unlike others in that phylum, crinoids’ tube feet lack a terminal sucker. Yet, they can still act as an adhesive filter (Byrne, M., and O'Hara, T. 2017) by producing mucous threads used in suspension feeding (Rutman, J., and Fishelson, L., 1969). C. multiradiatus have roughly six tube feet per mm, and each are an average length of 07.mm (Meyer, D. I., 1979).
 Figure 10: Tube feet extending out of the ambulacral groove of a pinnule on the arm of Capillaster multiradiatus
Figure 10: Tube feet extending out of the ambulacral groove of a pinnule on the arm of Capillaster multiradiatus
Cirri
Cirri are root-like projections from the bottom of the theca (part of the crown) on the centrodorsal of crinoids, shown in figure 11. Stalkless comatulids use the cirri instead of the stalk to attach the cup to substrate (Baumiller, T. K., 2008). In C. multiradiatus they are typically less than 25mm long, and made up of less than 25 segments known as cirrals (Messing, C. G., 2003). They are non-feeding appendages that are used only for attachment and not locomotion, like they can be in some species of comatulids (Fishelson, L., 1974).

Figure 11: The cirri of Capillaster multiradiatus extending from the bottom of the crown
|
|
|
Internal Anatomy | |
Water Vascular System
All echinoderms possess a water vascular system (WVS), and the crinoid WVS is very similar (Ruppert, E. E. et al. 2004). It is a collection of fluid-filled tubes and bladders, ending in numerous tube feet, and supports respiration and circulation (Fatherree, J. W. 2013). However, crinoids WVS has two main differences to the rest of the phylum; they lack a madreporite, and stone canals. Instead of a madreporite, often hundreds of tegmenal pores are present on the oral surface that lead into the perivisceral coelom. The ring canal gives rise to the stone canals which also lead into the perivisceral coelom (Ruppert, E. E. et al. 2004). It’s most likely that seawater enters via the perivisceral coelom and tegmenal pores and then is pumped into the WVS by the stone canals (Ruppert, E. E. et al. 2004).
From the central ring canal, a radial canal enters each one of the arms and extends a lateral canal into each pinnule (Ruppert, E. E. et al. 2004). The radial canals parallel the ambulacral grooves along the arms (Byrne, M., and O'Hara, T. 2017). Hydraulic pressure is generated by contracting muscle fibres connecting to the radial canal. This extends the tube feet which are situated off the lateral canals (Ruppert, E. E. et al. 2004).
Mutable tissue
Echinoderms have the ability to change the mechanical properties of connective tissues in their body, within just a few seconds (Wilkie, I. 2002). Mutable collagenous tissue (MCT) is present in some of the connective tissue of crinoids, meaning they’re able to adjust properties such as viscosity, stiffness, and tensile strength through active neural control (Baumiller, T. K., 2008). While the dermis contains muscles, nerves, and other cell types, its within the extracellular matrix that this stiffening occurs. There are two types of nerves present within the extracellular matrix, one that hardens and another that softens, and it appears that changes in calcium affect these nerves (Ruppert, E. E. et al. 2004).
This function is highly important; by stiffening structures such as their arms, crinoids are able to withstand high external forces, and destiffening means their body can move with minimal force (Baumiller, T. K., 2008). Crinoids can maintain their posture, particularly during feeding, by stiffening the MCT, and use a lot less energy than if they were to do so with muscle (Baumiller, T. K., 2008).
Body wall and Musculature
Crinoids have both a cuticle and an epidermis surrounding their body. The epidermis is only ciliated in the ambulacral grooves (Ruppert, E. E. et al. 2004). Below the epidermis is a connective-tissue layer that is continuous with the deeper connective tissue in the body. The cirri, arms, and pinnules are almost solid, filled with a series of thick, vertebra-like ossicles (Ruppert, E. E. et al. 2004).
Distinct musculature is confined mainly to the arms and pinnules (Ruppert, E. E. et al. 2004). Obliquely striated flexor muscles that run between the proximal edges of the arm and the pinnule ossicles flex the arms and pinnules and make them curl (Ruppert, E. E. et al. 2004). Muscular articulations allow mobility between the plates of the arms, meaning the arms can have flexible movement (Oji, T. and Okamoto. T., 1994). This is incredibly important as a crinoids’ arms perform feeding, and locomotion.
Ossicles of the arms are joined together by ligaments or muscles and ligaments (Byrne, M., and O'Hara, T. 2017). Contraction causes the arm to bend inwards (Byrne, M., and O'Hara, T. 2017), and elastic recoil of the interbrachial ligaments causes extension of the appendages (Ruppert, E. E. et al. 2004) When the muscles relaxes, ligaments can become rigid and allow the arm to maintain its extended feeding posture with little energy expenditure (Byrne, M., and O'Hara, T. 2017).
Identification of the species
Many different reports have been published regarding correct identification of feather stars (Clark, A. H. 1921, Pawson, D. L. 1972, Rowe, F. W. E, et. el. 19860. However, Summers et. al. 2017 produced a succinct and clear table outlining the diagnostic morphological characteristics of species in Comatulidae. They highlighted some of the main identification points, including an excentric mouth (not central), first syzygy at 3+4 on arms arising from IBr, and comb teeth that are single, triangular, confluent with outer edge of pinnule, and not tapering significantly distally, or tapering to a blunt tip (Summers, M. M. et. al 2017).
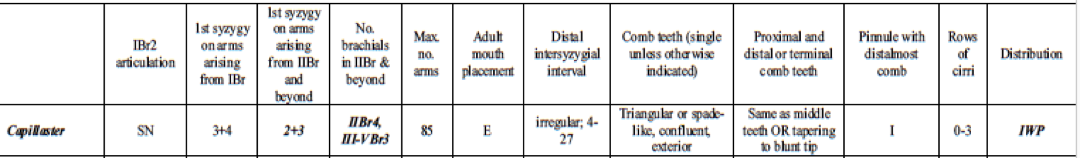
Table 1: Table explaining the diagnostic morphological characteristics of Capillaster multiradiatus. Image source: (Summers, M. M. et. al, 2017)
A syzygy is “where radiating ridges and grooves on the two joint faces appose each other so that the external suture resembles a perforated line” (Byrne, M., and O'Hara, T. 2017). Crinoids are modular and their skeletons have several repeated parts along their arms, so some of their forms are abbreviated (Byrne, M., and O'Hara, T. 2017). The abbreviation br is used for and individual brachial (one individual piece of skeleton), brachitaxes are abbreviated to Br, proceeded by a roman numeral indicating its position from the base of the ray, and followed by a number indicating the number of component ossicles (Byrne, M., and O'Hara, T. 2017). If a + is present, it indicates that there is a syzygy present between those two ossicles. For example, IIBr 4(3+4) indicates that this is the second brachitaxes on the arm (branching has occurred once), that there are four ossicles present in this brachitaxes, and that there is a syzygy between brachial three and four.
Having IIBr 4(3+4) is actually one of the species identifiers of Capilaster multiradiatus, the first syzygy at 3+4 on arms arising from IBr (from the second brachitaxes). Figure 12 demonstrates this syzygy.

Figure 12: The syzygy at IIBr 4(3+4) and the entire IIBr brachitaxes of the Capillaster multiradiatus specimen
Another identifiable characteristic is that of triangular, confluent, and exterior comb teeth on the pinnules, shown in figure 13.

Figure 13: A pinnule of Capillaster multiradiatus showing the comb teeth as triangular, confluent, and ending at a blunt tip
Digestive System
After food particles are caught by the tube feet and mucus and moved into the ambulacral groove, cilia transport it towards the mouth. It reaches the digestive system which is contained in the central disc, including a mouth, that leads into the oesophagus, and then into the intestine. The intestine does a complete circle around the inside of the calyx wall and then passes back up into the rectum and out of the anus (Ruppert, E. E. et al. 2004).
There is very limited information of the process of digestion in crinoids (Meyer, D. L. 1982), however there are claims that both extracellular and intracellular digestion occur in the intestine. Waste is excreted as large, compacted, mucus-cemented pellets, they fall from the anal cone and drop form the body (Ruppert, E. E. et al. 2004).
|
|
|
Physiology | |
Circulation
The perivisceral coelom is the main circulatory system within a crinoid, and exists in the central disc, arms, and pinnules (Ruppert, E. E. et al. 2004). Within the central disc, it surrounds the gut and the axial organ, which is a large blood vessel that holds epithelial tubes. Branches from this coelom enter the arms where a bilateral pair of oral coelomic cavities and unpaired aboral cavities exists. A branch from one oral and one aboral cavity extend into the pinnule united by canals. Flow is outbound from the disc in the aboral cavity, and inbound in the oral cavity (Ruppert, E. E. et al. 2004).
The oral hemal ring is the centre of the hemal system, and this gives rise to the axial organ and a network of hemal vessels in the coelomic mesenteries. Crinoids do not have a heart and the blood is colourless, yet the axial still organ pulses (Ruppert, E. E. et al. 2004).
Respiration and excretion
There is little information regarding respiration of crinoids, all that is known is that the tube feet are the primary sites of gas exchange. The large surface area of the arms means that any special respiratory surfaces are not required. There is also no special excretory organs of crinoids currently known(Ruppert, E. E. et al. 2004).
Autotomy and Regeneration
Crinoids have the ability to cast off a part of their body and then regenerate those parts back (Baumiller, T. K., and Gahn, F. G. 2012). Loss of body parts, including the appendices (arms, pinnules, cirri) and internal organs or whole visceral mass, can occur via self induced loss or by physical disturbances, wave action, entanglement, or unfavourable conditions causing stress. In some experiments, crinoids have been able to regenerate their body parts even when the body was halved, or reduced to only one fifth (Carnevali, C. 2006).
The nervous system plays an important role in regeneration, and some of the main cellular elements responsible for the regrowth of appendices and visceral mass include amoebocytes, coelomocytes, granulocytes, phagocytes (Meyer, D. 1985). Autotomy and subsequent regeneration are very important when crinoids are dealing with predation (Baumiller, T. K., 2008) with regeneration occurring quite quickly after disturbance events (Meyer, D. 1985).
Larvae are also able to undergo partial regeneration of some parts of their body, but it isn’t a commonly recorded event (Carnevali, C. 2006).
Capillaster multiradiatus lost many pinnules and segments of arms during this observational period, and showed incredibly quick regeneration of two new arms, as seen in figure 14.
Figure 14: Two new arms growing from one of the radials of the central disc of Capillaster multiradiatus
|
|
|
|
Biogeographic Distribution | |
Figure 15: Map showing occurrences of Capillaster multiradiatus in all observed locations. The lighter yellow the dot, the fewer occurrences; whereas orange dots signify higher observation numbers. Image source: gbif.org
Capillaster multiradiatusis found in many tropical and sub-tropical locations, but its main range is the tropical Indo-western Pacific, as shown by the dots in figure 15. It exists in northern Australia from Fremantle in Western Australia, to Lady Musgrave Island in Queensland, in the west it reaches East Africa and the Red Sea, and in the east extends to New Caledonia and Vanuatu. To the north, this species has been found in the East China Sea, west of Amami-Oshima, Japan, and Chuuk Atoll (Summers, M. M. et. al 2017).
|
|
|
Evolution and Systematics | |
Fossil Record
There are abundant skeletal remains found within the fossil record, with more than 6000 fossil species having been discovered and described (Byrne, M., and O'Hara, T. 2017). During the Early Ordovician, around 485 million years ago, recognisable crinoid forms appeared. During the Palaeozoic, these species underwent several major radiations, and evidence appeared of them becoming major carbonate-producing organisms. The remains of crinoid skeletons are one of the primary components of carbonate shelves in many Palaeozoic and Mesozoic settings. As well as this, crinoid fossils are also extensively known from Australia (Byrne, M., and O'Hara, T. 2017).
Evolution
The extensive fossil record of not only crinoids, but also echinoderms, reveals both evolutionary and phylogenetic relationships among the phyla and families. Many morphological adaptations have occurred since the first crinoids; this includes the centrodorsal formation and the morphology of the cup [31]. Comatulids first appeared in the Jurassic, although their diversification in the Upper Jurassic was far from an explosive radiation. Any increase in the number of specimens only occurred with the appearance of suitable reef habitat (Hess, H. 2014).
During the Mesozoic, intensified predation pressure from a rise in modern durophagous (eating exoskeleton bearing organisms) predators was likely a selective factor for the predator-resistant characteristics in comatulids today (Meyer, D. 1985). Many of the modern species’ mechanisms for coping with predation, including free-movement, a nocturnal lifestyle, and regeneration, have likely evolved as a direct response to the diversification and intensified predation from mobile predators during this time period (Meyer, D. 1985).
Phylogeny
Crinoids belong to the phylum Echinodermata, which include the classes Crinoidea (feather stars), Asteroidea (sea stars), Ophiuroidea (brittle stars), Echinoidea (sea urchins), and Holothuroidea (sea cucumbers) (Ruppert, E. E. et al. 2004), the family phylogenetic tree is shown in figure 16.
Figure 16: Phylogenetic tree of the Echinoderms showing the relationship among the classes. Image source: Baumiller, T. K., 2008.
Throughout the past 200 or so years, the phylogeny and nomenclature of Crinoidea has changed significantly (Summers, M. M. et. al 2017). It is agreed that all extant crinoids can be placed in the subclass Articulata (Byrne, M., and O'Hara, T. 2017). The four major clades within Crinoidea are Isocrinida, Cyrtocrinida, Hyocrinida, Comatulida, of which Capillaster multiradiatus falls into the latter. Nested into Comatulida are two stalked groups, Guillecrinidae and Bourgueticrinina, providing evidence for the idea that the Bourgueticrinines arose from feather stars ancesors (Byrne, M., and O'Hara, T. 2017).
The family Comatulidae is divided into two subfamilies, six tribes, 22 genera and roughly 100 species; of which C. multiradiatus falls into subfamily Comatulinae, tribe Capillasterini, and genus Capillaster (Summers, M. M. et. al 2017).
|
|
|
Conservation and Threats | |
Status
Capillaster multiradiatushasn’t been reviewed by the IUCN for its conservation status, but it is likely not under immediate threat from extinction.
Threats
There are few threats facing C. multiradiatus,particularly given that it has no highly lethal predators (Baumiller, T. K., and Gahn, F. G. 2012). Into the future increases in atmospheric CO2 will cause the worlds oceans to warm and also become far more acidic (Dupont, S. et. al 2010). Since the internal skeleton of crinoid adults is formed via calcite deposition, there is a likelihood that crinoids, and all echinoderms, may be a key group impacted by climate change (Dupont, S. et. al 2010).
|
|
|
References | |
Alender, C.B. et al. 1966. Physiology of Echinodermata. John Wiley & Sons, Inc, United States of America
Baeza, J. A. 2011. Squat lobsters as symbionts and in chemo-autotrophic environments. The Biology of Squat Lobsters 8, 249-270.
Baumiller, T. K., 2008. Crinoid Ecological Morphology. Annual Review of Earth and Planetary Sciences, 36, 221-249
Baumiller, T. K., and Gahn, F. G. 2012. Reconstructing predation pressure on crinoids: estimating arm-loss rates from regenerating arms. Paleobiology, 39(1), 40-51
Bradbury, R. H. Reichelt, R. E., Meyer D. L., and Birtles, R. A. 1987. Patterns in the distribution of the crinoid community at Davies Reef on the central Great Barrier Reef. Coral Reefs 5(4), 189-196.
Byrne, M., and O'Hara, T. 2017, Australian Echinoderms: Biology, Ecology and Evolution, CSIRO PUBLISHING
Carnevali, C. 2006. Regeneration in Echinoderms: repair, regrowth, cloning Invertebrate. Survival Journal 3(1).
Clark, A. H. 1921. A Monograph of the Existing Crinoids. Volume 82 of Bulletin United States National Museum. Govt. Print. Off.
Deheyn., Lyskin, S., and Eeckha, I. 2006. Assemblages of symbionts in tropical shallow water crinoids and assessment of Symbionts host specificity. Symbiosis, 42(3), 161-168
Dupont, S., Ortega-Martínez, O. and Thorndyke, M. 2010. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19(3) 449-462.
Encyclopedia of Life, 2018, Allogalathea elegans (Adams, A and A. White, 1848), Elegant Squat Lobster, http://eol.org/pages/2869578/overview, last viewed: 1/6/18
Fabricius, K. E. 1994. Spatial Patterns in Shallow-water Crinoid Communities on the Central Great Barrier Reef. Australian Journal of Marine and Freshwater Research 45(7), 1225 – 1236.
Fabricius, K. E. and Dale, M. B. 1993. Multispecies Associations of Symbionts of Shallow Water Crinoids of the Central Great Barrier Reef. Coenoses 8(1), 41-52
Fatherree, J. W. 2013. Aquarium Invertebrates: A Good Look at the Crinoids, Advanced Aquarist 7(1), accessible via https://www.advancedaquarist.com/2013/7/inverts
Fishelson, L., 1974. Ecology of the Northern Red Sea Crinoids and Their Epi- and Endozoic Fauna. Marine Biology, 26(2), 183-192.
gbif.org, 2018. Capillaster multiradiatus (Linnaeus, 1758) stdterms.in GBIF Secretariat (2017). GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei accessed via GBIF.org on 2018-06-01.
Hess, H. 2014. Origin and radiation of the comatulids (Crinoidea) in the Jurassic. Swiss Journal of Palaeontology, Volume 133(1), 23–34
Marshall, J. I. and Rowe, F. W. E. 1981. The Crinoids of Madagascar. Bulletin du Muséum national d'histoire naturelle 3(2), 379-413
McEdward, L. R., and Miner, B. G., 2001. Larval and life-cycle patterns in echinoderms. Canadian Journal of Zoology, 79(7), 1125-1170.
Meyer, D. L. 1979. Length and Spacing of the Tube Feet in Crinoids (Echinodermata) and Their Role in Suspension-Feeding. Marine Biology, 51(4), 361–369.
Meyer, D. L. 1982. Food composition and feeding behaviour of sympatric species of comatulid crinoids from the Palau Islands (Western Pacific), p. 43–49. In Lawrence, J. M. (ed.), Echinoderms: Proceedings of the International Conference, Tampa Bay. A. A.
Meyer, D. L. 1985. Evolutionary Implications of Predation on Recent Comatulid Crinoids from the Great Barrier Reef. Paleobiology, 11(2), 154-164.
Messing, C. G., 2003. Three new species of Comasteridae (Echinodermata, Crinoidea) from the tropical western Pacific. Zoosystema 25(1), 149-162.
Messing, C. G. and Tay, T. S. 2016. Extant Crinoidea (Echinodermata) of Singapore. Raffles Bulletin of Zoology 34(2), 627-658
Oji, T. and Okamoto. T., 1994. Arm Autotomy and Arm Branching Pattern as Anti-Predatory Adaptations in Stalked and Stalkless Crinoids. Paleobiology 20(1), 27-39.
Online Zoological Collections of Australian Museums. 2005. Occurrence record: Invertebrates:F112065, Preserved specimen of Capillaster multiradiata | feather star recorded on 2005-12-02. https://ozcam.ala.org.au/occurrences/7334caf1-bedf-4a9f-976d-6822371481c2, last viewed: 1/6/18
Pawson, D. L. 1972. Monograph of Shallow-water Indo-west Pacific Echinoderms. The Quarterly Review of Biology 47(1), 111
Rowe, F. W. E., Hoggett, A. K., Birtles, R. A., and Vail, L. L. 1986. Revision of some comasterid genera from Australia (Echinodermata: Crinoidea), with descriptions of two new genera and nine new species, Zoological Journal of the Linnean Society, 86(3), 197–277.
Ruppert, E. E., Fox, R. S. and Barnes, R. D. 2004. Invertebrate Zoology: A Functional Evolutionary Approach, 7th ed. Australia. Brooks/ Cole Cengage Learning,.
Rutman, J. L., and Fishelson, L., 1969. Food composition and feeding behavior of shallow-water crinoids at Eilat (Red Sea). Marine Biology, 3(1), 46–57.
Rutman, J. L., and Fishelson, L. 1985 Comparison of Reproduction in the Red Sea Feather-Stars Lamprometra klunzingeri (Hartlaub), Heterometra savignii (J. Muller) and Capillaster multiradiatus (L.). Echinodermata (Proceedings of the 5th International Conference, Galway, 24-29 September 1984), 195-201
Summers, M. M., Messing, C. G., and Rouse, G. W. 2017. The genera and species of Comatulidae (Comatulida: Crinoidea): taxonomic revisions and a molecular and morphological guide. Zootaxa, 4268(2), 151-190
Vail, L. 1987. Diel patterns of emergence of crinoids (Echinodermata) from within a reef at Lizard Island, Great Barrier Reef, Australia. Marine Biology, 93(4), 551-560.
Wilkie, I. 2002. Is muscle involved in the mechanical adaptability of echinoderm mutable collagenous tissue? Journal of Experimental Biology 205(2), 159-65.
|
|
|
|
|